Force Spectroscopy in Molecule Binding
Gold-coated silicon substrates can promote molecule attachment and result in self-assembled monolayers (SAMs) in AFM, force spectroscopy, and mass spectroscopy applications. The high-purity and uniformity of gold-coated substrates provide the ideal surface for characterization methods.
A research study conducted at the University of California, Lawrence Livermore National Laboratory, investigated the binding capabilities of a protein and ligand utilizing atomic force microscopy (AFM) along with polymer tethers. Platypus Technologies’ gold-coated silicon substrates were used to create a functionalized surface enabling the study of protein and ligand interactions.
The bond rupture force was investigated between concanavalin-A protein (ConA) and mannose. ConA was selected for evaluation due to its role in carbohydrate-binding. Carbohydrate-binding molecules possess distinguished chemical and physical properties that can be readily manipulated through controlled binding with a ligand. The ligand, a molecule that facilitates binding to another molecule, selected for binding was a sugar known as mannose. The investigation of rupture force between ConA and mannose can be used to study diseases and promote the discovery of new biological advancements.
Force spectroscopy was used to reduce nonspecific binding of biological molecules. Molecules were attached to an AFM cantilever in order to probe the substrate surface. The deflection of the cantilever was examined in order to indicate changes in deflection and the force needed to create a bond rupture. Bond rupture is determined through calculating the distance the polymer is stretched before approaching rupture. In this experiment, the binding species were attached to polymer tethers.
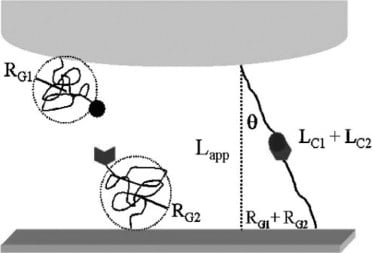
As opposed to a single-tethered system, researchers applied a double tethering system. A double tethering system allowed the protein to attach to the AFM top and the ligand to attach to the gold substrate. Creating localized specific and nonspecific binding helped to eliminate the possibility of non-specific binding. In addition, this method improves the overall accuracy of force measurements.
Silicon nitride cantilevers were salinized to reduce the number of active groups on the AFM tip prior to being immersed in a buffer solution containing ConA. Platypus gold-coated silicon substrates were then immersed in ethanol to produce SAMs that were then immersed in the mannose solution. Thus, creating a mannose functionalized substrate.
AFM measurements were then taken between the tethered ConA protein and mannose substrate. Three length scales were identified as adhesive interactions.
The rupture force was determined by the tip to substrate distance. Section C of the figure above illustrates the protein and ligand interaction between 33 and 43mm. This location represents the initial breaking of the protein-carbohydrate bond between ConA and mannose. This location shows a significant increase in length, indicating bond rupture. Results were further confirmed with the addition of a mannose buffer that served as a protein-ligand bonding inhibitor. The previously indicated adhesive interactions between 33 and 43mm were diminished through the addition of the blocking agent, whereas other regions were unaffected.
In conclusion, AFM techniques and gold-coated silicon substrates can provide encouraging results when studying nonspecific binding.
Resources:
Force Spectroscopy of the Double-Tethered Concanavalin-A Mannose Bond – ScienceDirect