Chronic Inflammation and Migration of Colon Cancer Cells
In a new research study from Tokyo Medical and Dental University in Japan, scientist investigated the influence of chronic inflammation on infectious phenotypes encountered with ulcerative colitis (UC), a disease of the bowels.
Gene mutations are commonly detected in the endoscopic examination of UC. The difficulty with identifying colitis-associated cancers (CACs) is caused by the obstacle of chronic inflammation. Currently, the effect of potential inflammation on sporadic neoplasms has not yet been identified. Sporadic neoplasms provide a more accurate diagnosis of UC compared to CAC identification. In this study, a long-term inflammatory model of a correctional cancer cell line (LS174T) was created.
LS174T, colon adenocarcinoma-derived cell line, was cultured with flagellin, interleukin, and tumor necrosis factor (TNF). Flagellin is primarily responsible for the adhesion and invasion of host cells. Interleukin modulates cell behavior through cellular signaling. TNF is secreted by inflammatory cells and contributes to cell proliferation and differentiation.
CRISPR technology was then used to mutate exon 10 of TP53 in LS174T cells. The resulting mutated cells had a lower expression of P53 downstream genes. Real-time polymerase chain reaction (PCR) tests were used to show the expression levels of cancer invasiveness related genes and analyze cell cycle progression genes and stem cell marker genes. An MTS assay measured cell proliferation to indicate the ratio of absorbance in the cell line.
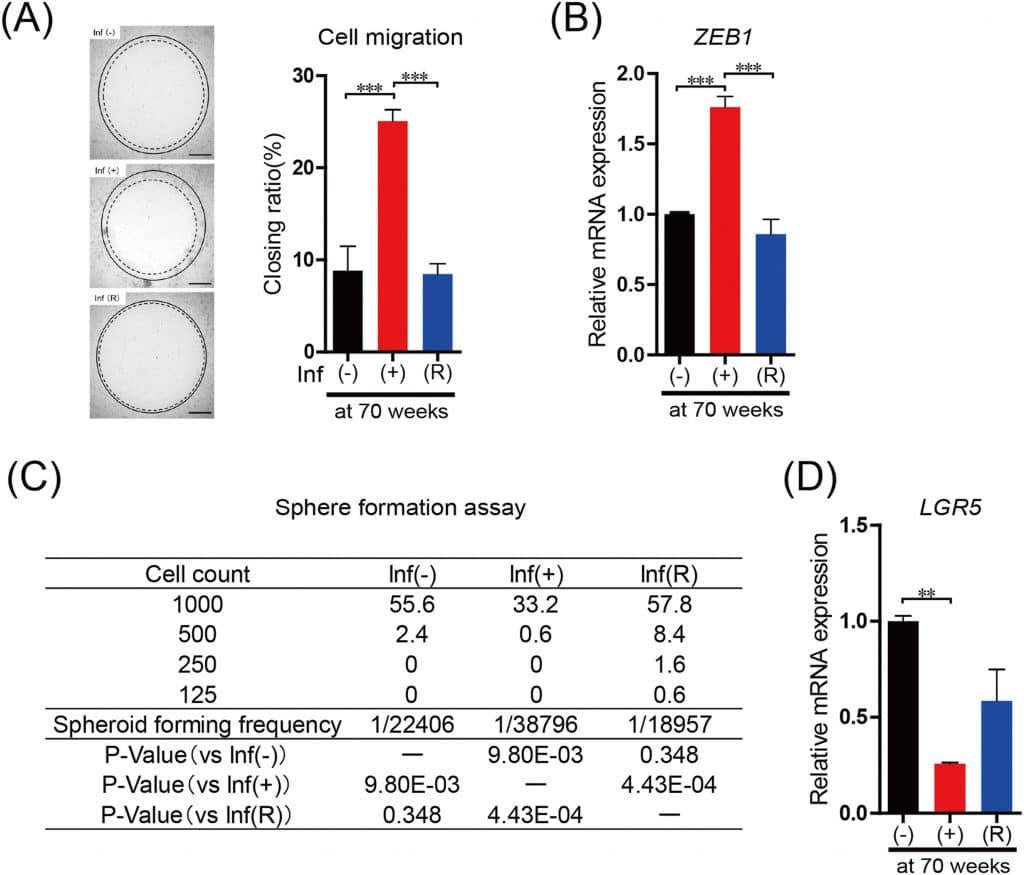
Platypus Technologies Oris Cell Migration Assay was used to further evaluate malignant phenotypes by measuring the invasiveness of the LS174T cells. Platypus Technologies Oris Cell Migration Assay allow for reproducible, accurate, and precise assessment of cellular migration.
The results generated from the cell migration assay and other tools indicated that LS174T cells that were exposed to inflammatory reagents had a higher induction of interleukin-8 (IL-8). IL-8 is a chemoattractant cytokine that activates neutrophils in inflammatory regions. The experiments preformed in this research indicated the effects of long-term inflammation on LS174T cells. During a 60-week time span, a downstream gene NF-kB p65, was continuously produced indicating long-term inflammation. It was found that removing the inflammatory stimuli often restored normal levels under varying analyses. NF-kB signaling of LS174T cells induced by chronic inflammation was restored to normal levels after removal of the inflammatory stimuli. In addition, the proliferation and viability of LS177T cells were also restored to normal levels after removing the inflammatory stimuli. Therefore, showing that chronic inflammation promoted the migration and invasion of LS174T cells.
Invasiveness was restored to normal levels after inflammatory stimuli removal. This chronic inflammation prevented the production of Lgr5, a cancer stem cell marker. Invasiveness was enhanced in TP53 mutated cells.
In summary, this research illustrated that removing inflammatory stimuli allowed invasiveness to return to normal levels. Thus, revealing a high cellular plasticity in single colorectal cancer cells.
References: