Electrochemical Analysis of Dyes with Gold Electrodes
Gold-coated silicon wafers provide inherent electrical properties that can be used in electrochemical applications. Platypus Technologies silicon substrates are carefully engineered to serve as high-performance electrodes.
A new publication from Clemson University in South Carolina describes an electrochemical analysis method to determine the amount of active charge material present within in a Prussian Blue Analogue (PBA) called nickel hexacyanoferrate (Ni-HCF). PBAs are promising materials for the development of new battery technologies due to their unique open crystalline structure and tunable conductivity. This structure can be modified to improve conductivity of the material.
The paper describes an electrochemical method where a material (Ni-HCF) is produced directly on an electrode. Gold-coated silicon wafers from Platypus Technologies were used in order to create an active electrode for this study. The authors aimed to determine the amount of material that is active in the charge storage of Ni-HCF.
Ni-HCF was produced with a two-step electrochemical process onto the gold-coated silicon wafer. Charge capacity was found by cyclic voltammetry (CV), a technique that is useful when evaluating oxidation-reduction reactions and can be used to measure the active material present in a sample.
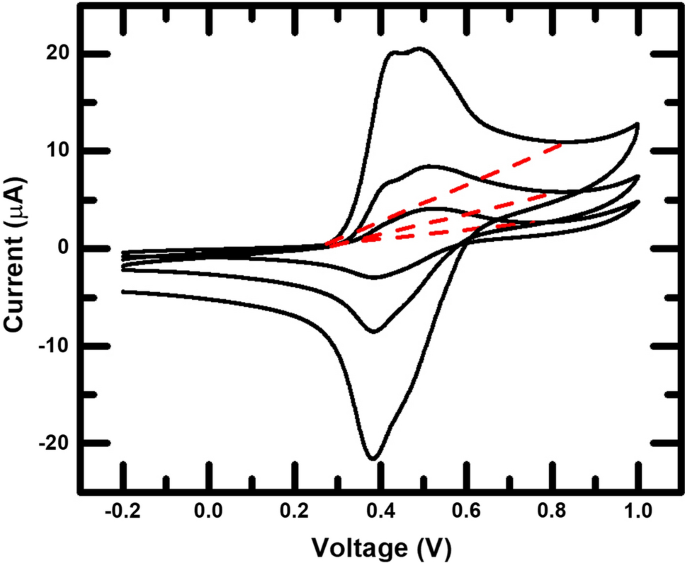
Platypus Technologies gold-coated silicon wafer served as both the substrate and the active electrode for the experiment. Nickel was first deposited onto the gold surface with DC potential amperometry, where voltage was adjusted until proper charge passed through the electrode. Next, cyclic voltammetry was used to measure the current as the electrode potential varied. The current measured as a function of voltage during film fabrication is shown below, illustrating the shifts in current at 0.4 Volts. The increasing peaks on the spectra indicate surface binding of the Ni-HCF film.
The authors conducted additional x-ray spectroscopy measurements on the gold-coated wafers functionalized with Ni-HCF. These measurements were useful to characterize the stoichiometry of the PBA.
Further investigation may be conducted to evaluate the possibility of sample-to-sample variation caused by sample roughness and kinetic differences. Nonetheless, a non-destructive comparative electrochemical process was established in order to measure the amount of Fe present in a PBA sample. This work could be applied to increase the efficiency of PBA samples to create improved battery-like materials.
In conclusion, gold-coated silicon wafers by Platypus Technologies were used to develop and characterize novel materials for battery technologies.
Resources: