Cell Invasion Assays for Cancer Research
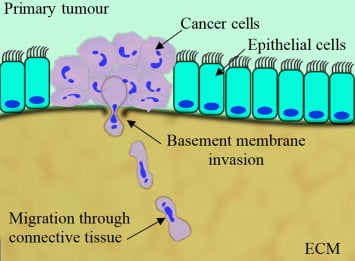
Cell invasion across the basement membrane is an important step in cancer metastasis. Metastasis occurs when cancer cells pass through the basement membrane of the organ where they originated, and subsequently spread into different organs of the body, where they form secondary tumors
Cell invasion across the basement membrane is an important step in cancer metastasis. In this blog, we discuss cell invasion assays and their application for cancer research. Metastasis occurs when cancer cells pass through the basement membrane of the organ where they originated, and subsequently spread into different organs of the body, where they form secondary tumors [1].
Cell invasion assays are important tools for cancer research. Scientists studying cancer perform cell invasion assays to measure the cell movement through an extracellular matrix. By varying the conditions of the cell culture (e.g. adding new drugs), scientists can identify conditions that prevent or accelerate cell invasion. Through these studies, new treatments for cancer can be identified for potential therapeutic use.
SIMPLE & RELIABLE CELL ASSAYS FOR BREAKTHROUGH SCIENCE
To accelerate scientific breakthroughs in cancer research, Platypus Technologies has introduced a simple, reproducible assay platform to study cell invasion. The OrisTM platform comprises 96-well plates with removable “stoppers” that create a central cell-free detection zone. For a cell invasion assay, this detection zone can be filled with an extracellular matrix of choice (e.g. collagen, basement membrane extract (BME), hyaluronic acid, hydrogels, etc.). The cells invade into the matrix in the detection zone and the extent and rate of cell invasion is easily quantified, in real-time, using a plate reader or from microscope images. Compared to other approaches, the OrisTM platform offers unique advantages for studying cell invasion:
- Very simple set-up and straightforward data interpretation
- Accurately identify specific drugs or conditions that inhibit or stimulate cell invasion
- Reproducible detection zone enables high accuracy of results
- Supports real-time and end-point measurements of cell invasion
- Easy analysis:
- Use the OrisTM Detection Mask to quantify invasion using plate readers
- Doing image analysis? No stains or mask required
- Results are physiologically relevant: cells invade directly into the extracellular matrix; no artificial membranes are needed.
EXAMPLE OF CELL INVASION ASSAY
Below, an experiment illustrates the use of the OrisTM platform for studying cell invasion through basement membrane extract (BME). The main materials required to perform this assay include: (1) OrisTM cell migration assay coated with BME, (2) BME solution, (3) cells and (4) cell culture media. HT1080 fibrosarcoma cells were live-stained and cultured on the OrisTM plate coated with BME. Following a period of incubation to allow for cell attachment, the stoppers that create the central cell-free detection zone were removed, and the wells of the plate were filled with chilled BME solution. Subsequently, the cells embedded in the BME were incubated at 37 °C. Images of the cell culture were collected at various intervals and analyzed with ImageJ.
[SEE DETAILED PROTOCOL FOR ORISTM CELL MIGRATION ASSAY]
The figure below captures representative images of the cell culture before removal of the stoppers, and 40 hours of incubation of the cells in BME.
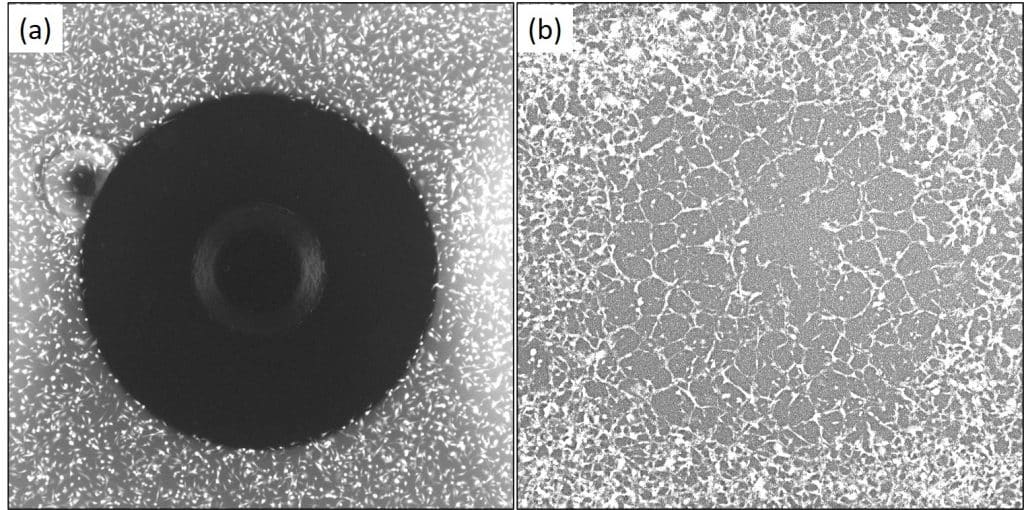
As shown in these images, the OrisTM platform allowed for the cells to attach around the circular cell-free detection zone. This detection zone, filled with BME, makes it easy to characterize cell invasion: cells that move into the circular detection zone can be readily quantified.
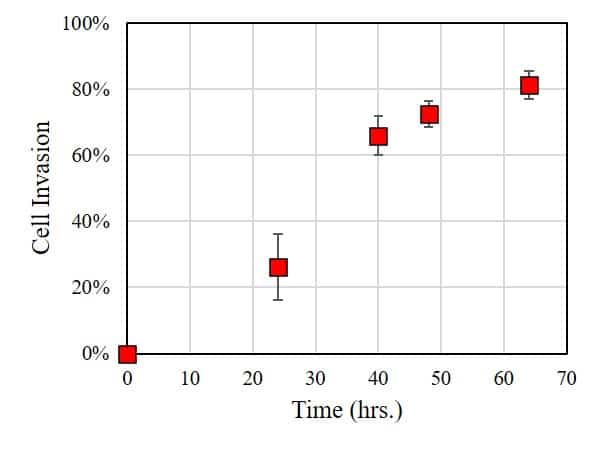
In this case, the extent of cell invasion was characterized by using ImageJ to measure the area of the cell-free zone: the smaller the area, the larger the extent of cell invasion into the BME. As shown in the figure below, within 24 hours of incubation, a significant number of cells invaded into the BME. Longer incubation times result in significantly higher rates of cell invasion.
CONCLUSION
This experiment demonstrates that HT1080 exhibits significant cell invasion into the basement membrane. Follow-up experiments could explore parameters that influence cell invasion such as (i) stiffness of BME, (ii) drugs and concentration of drugs present in the culture media, or (iii) presence of secondary cell culture (co-culture) present in the BME. In conclusion, the OrisTM platform provides a simple and reliable platform for carrying cell invasion assays.
REFERENCES:
[1] T. A. Martin et al. “Cancer Invasion and Metastasis : Molecular and Cellular Perspective” In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013. LINK
[2] M. Malboubi et al. “An open access microfluidic device for the study of the physical limits of cancer cell deformation during migration in confined environments” Microelectron. Eng. vol. 144, 16 Aug. 2015 pp. 42-45. LINK