Unlocking Alzheimer’s Molecular Secrets with Infrared Nanospectroscopy
Alzheimer’s disease is a devastating condition characterized by memory loss and cognitive impairment, causing immense suffering for patients and their families. One of the main causes of Alzheimer’s is the aggregation of a protein called amyloid-β (Aβ42) in the brain, leading to the formation of toxic structures. Scientists have been working tirelessly to understand the molecular basis of this disorder and develop treatments that can stop or reverse the aggregation process. In a groundbreaking study, researchers used infrared nanospectroscopy and ultra-flat gold to explore the interactions between Aβ42 aggregates and a small molecule inhibitor.
Infrared Nanospectroscopy: A Game-Changer for Drug Discovery
Infrared nanospectroscopy is a cutting-edge technique that combines atomic force microscopy (AFM) with infrared (IR) spectroscopy, allowing scientists to examine the structure and composition of tiny particles at the nanoscale. This powerful tool is revolutionizing the way we study complex biological systems, as it can provide detailed information about individual molecules and their interactions.
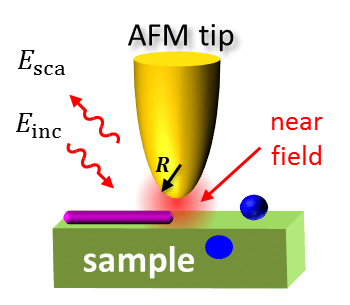
In this study, the researchers utilized infrared nanospectroscopy to examine the effect of a small molecule inhibitor, bexarotene, on the aggregation of Aβ42. Bexarotene is an FDA-approved anti-cancer drug that has been shown to significantly delay Aβ42 aggregation and reduce its toxicity in animal models. By understanding how bexarotene interacts with Aβ42 aggregates, scientists hope to develop more effective treatments for Alzheimer’s disease.
Ultra-Flat Gold: The Key to High-Resolution Imaging
To get the most accurate results, the researchers needed a substrate that would allow them to obtain high-resolution images of the Aβ42 aggregates and their interactions with bexarotene. This is where ultra-flat gold from Platypus Technologies played a crucial role.
Ultra-flat gold is a specially designed substrate with an incredibly smooth surface, ideal for high-resolution imaging techniques like AFM. By using ultra-flat gold, the scientists were able to obtain detailed images of the Aβ42 aggregates, revealing key insights into their structure and interaction with bexarotene.
Key Findings: How Bexarotene Interacts with Aβ42 Aggregates
The researchers discovered that bexarotene interacts with Aβ42 aggregates through a single hydrogen bond involving the carboxyl group of the molecule. This interaction is critical for inhibiting the aggregation of Aβ42, as a derivative of bexarotene with a methyl ester group instead of the carboxyl group did not show the same inhibitory effects. This finding highlights the importance of understanding the precise molecular interactions between potential drug candidates and their targets.
The Future of Alzheimer’s Disease Research and Treatment
This study demonstrates the incredible potential of infrared nanospectroscopy and ultra-flat gold in advancing our understanding of the molecular mechanisms behind Alzheimer’s disease. By providing detailed information about the interactions between aggregating proteins and therapeutic compounds, researchers can develop more targeted and effective treatments for this devastating condition.
In conclusion, infrared nanospectroscopy and ultra-flat gold are transforming the field of drug discovery for Alzheimer’s and other protein misfolding diseases. By offering insights into the molecular interactions between potential treatments and their targets, these cutting-edge technologies are paving the way for more effective therapies and, ultimately, hope for millions of patients and their families.