Improved sensors for water contaminants using Au(111)
New research published in the Journal of the American Chemical Society, led by Professor Fernando Garzon of the University of New Mexico, demonstrates a novel strategy to improve sensors for water contaminants. The new approach involves using a thin films of highly oriented gold Au(111) on an electrode to enable redesign of the sensing surface and enhance its sensitivity.
New approach involves using thin films of Au(111) to enhance sensitivity.
In the article, Professor Garzon and his team describe techniques of cyclic voltammetry and electrochemical stripping voltammetry to investigate the adsorption and desorption of aqueous arsenic, As(III), on the surface of gold electrodes.
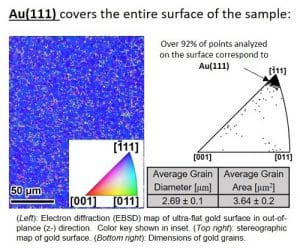
The Au(111) surface is highly ordered and flat, which has made it one of the most studied surfaces in materials science. The atomic-scale structure enables researchers to use their knowledge of chemistry and quantum mechanics to understand how electrons move around the surface, giving them insight into how they can improve its properties for different applications.
The researchers compared the performance of highly oriented gold, Au(111), from a single crystal with thin film surfaces fabricated by Platypus Technologies. They found these surfaces have improved detection capabilities for arsenic in water.
Electrochemical sensors can be used to rapidly detect contaminants in water sources.
Electrochemical sensors are made up of an electrode, which acts as a conductor between the water and the measuring device, and a sensing material that recognizes the contaminants present in the sample. These sensors can be used to rapidly detect contaminants in water sources. These types of sensors are low cost and compatible with portable electronics. They also have many advantages compared to other types of chemical sensors:
- Their reaction is fast and robust. When the target analyte (e.g. arsenic) comes into contact with the gold, the electrodes produce an electrical signal. This signal can be measured over time to determine how much of the target analyte there is in the water sample.
- Easily scalable. Electrochemical sensors can be mass produced on a large-scale using techniques such as printing, molding, and microfabrication.
- Low cost. The materials required for electrochemical sensors are inexpensive and widely available; therefore, the cost of making them is low as well. Furthermore, their small size makes it easy for users to carry around multiple units at once without issue or bulkiness in their pockets or bags.
Platypus Technologies offers ultra-flat surfaces of Au(111)
With a standard methods of fabrication, Platypus Technologies fabricates ultra-flat surfaces of Au(111) that are nearly atomically flat, with a deviation from the ideal surface being less than 1-nm. This is important for any application that requires precise control over the orientation of molecules or atoms on the surface. For example, these ultra-flat surfaces are well suited for fabricating sensors for detecting water contaminants, as well as ultra-sensitive sensors that detect small quantities of glucose and other organic compounds in body fluids such as urine and blood plasma.
Conclusion
In conclusion, the authors demonstrate a design for electrochemical sensors that is highly sensitive for arsenic, one of the deadliest toxins in water resources. The new platform should enable more rapid and accurate detection of contaminants in real-world settings. To learn more about this article, visit https://pubs.acs.org/doi/abs/10.1021/acs.jpcc.2c05541