The Role of Cell Migration Assays in Studying Heart Disease
Aneurysms are potentially life-threatening conditions caused by thinning of the blood vessels, allowing the arteries to bulge out abnormally. To elucidate the molecular underpinnings of congenital aneurysms, researchers have turned to cell migration assays, employing them as valuable tools in their investigations. This blog post explores the findings from such studies.
The epithelial-to-mesenchymal transition (EMT) is a biological process that occurs during embryonic development, wound healing, and in disease processes such as cancer. It involves the transformation of static, polarized epithelial cells into mobile, invasive mesenchymal cells. This transition, specifically the role it plays in congenital aneurysms, forms the cornerstone of the research published by scientists in Sweden.
In this study, researchers utilized aortic tissue samples from patients undergoing open-heart surgery to compare the types of EMT associated with two different heart valve configurations: bicuspid aortic valve (BAV) which is abnormal and associated with congenital heart disease, and tricuspid aortic valve (TAV), which is the normal configuration.
They found that BAV-associated aortopathy was linked to an enrichment of EMT genes related to endocardial cushion formation, a critical process in heart valve development. These BAV vascular smooth muscle cells were observed to be less proliferative and migratory compared to their TAV counterparts.
Intriguingly, these findings suggest different EMT types are associated with BAV and TAV. The distinct phenotypes hint at separate pathological mechanisms, marking a significant departure from traditional understandings of EMT. This is where cell migration assays come into play, serving as crucial instruments in examining these phenotypic differences.
The Oris cell migration assay, used in this study, is a robust and innovative method for measuring cell migration and proliferation in real-time. This assay highlighted the stark difference in the proliferative and migratory behaviors of BAV and TAV aortic smooth muscle cells. BAV muscle cells exhibited lower cell migration and proliferation compared to normal TAV cells.
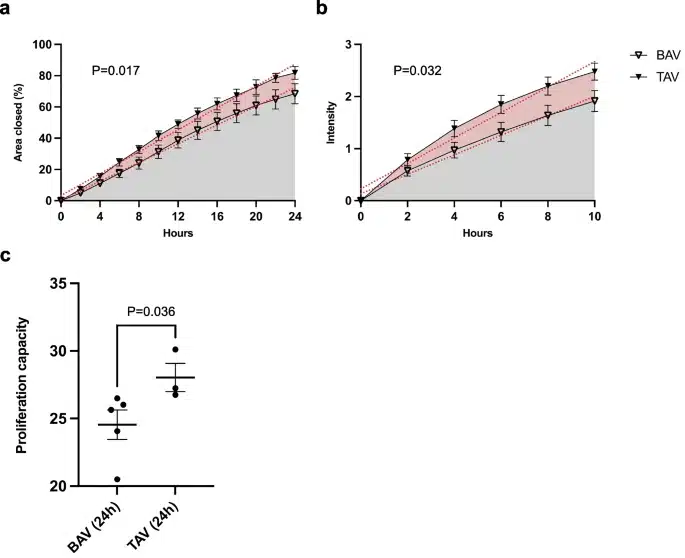
These findings have potential therapeutic implications, underscoring the importance of developing therapies tailored to the specific cell phenotypes.
Cell migration assays such as the Oris assay have proven instrumental in deepening our understanding of BAV-associated aortopathy. By enabling researchers to monitor cell migration and proliferation, these assays provide critical insights into the underlying pathological mechanisms of congenital aneurysms, offering hope for more personalized and effective therapeutic strategies.
In conclusion, cell migration assays represent a promising avenue for unraveling the mysteries of congenital heart defects like BAV. As we continue to refine these techniques and deepen our understanding, we move closer to an era of precision medicine where treatments are designed around the unique molecular signatures of each patient’s disease.
Learn more about Oris Cell Migration Assays.