Reimagining Cardiovascular Disease Treatment with Fibroblasts
The exciting realm of mesenchymal-to-endothelial transition (MEndoT), though controversial, is an area of research that could radically transform our approach to treating cardiovascular diseases. Key to this is understanding the potential role of fibroblasts – a type of cell known for its role in tissue homeostasis and disease – in the formation of new blood vessels. In this journey of discovery, the Oris Universal Cell Migration Assembly kit has proven to be a critical tool.
The Potential of Fibroblasts in Tissue Engineering
Fascinating research has been carried out into the endothelial differentiation capacity of human lung fibroblasts, specifically MRC-5 cells. The researchers’ goal was to investigate whether these fibroblasts could become endothelial cells (ECs) – the very cells instrumental in the formation of our blood vessels. The cells were cultured on top of, or embedded within, a Matrigel hydrogel. In response to angiogenic signals, these fibroblasts transformed into capillary-like networks, and at higher concentrations, they even invaded the Matrigel, forming stem-cell-like spheroids that sprouted to form 3D connective-tissue networks.
This finding is a significant milestone in the field of vascular tissue engineering. The capability to create bio-compatible vessel grafts that can grow, remodel, and repair in the patient’s body is a game-changer. However, there have been significant hurdles to clear. The process of harvesting autologous ECs – cells from the patient’s own body – is technically challenging, and the limited source and proliferative capacity of these cells make the process even more arduous. The potential of fibroblasts to transform into ECs could provide the key to overcoming these challenges.
The Role of Cell Invasion Assays
To delve deeper into this, the researchers utilized the Oris Universal Cell Migration Assembly kit to study MRC-5 cell invasion. This tool was instrumental in uncovering the behavior of the fibroblasts within the Matrigel matrix.
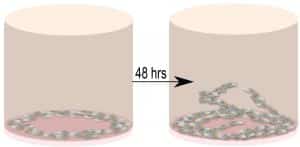
The researcher’s method for investigating cell invasion using the Oris Universal Migration kit is as follows:
“Each 96-well plate of the kit contained cell-seeding stoppers (2 mm in diameter) for creating a detection zone at the center of each well. The wells of the 96-well plate were coated with 50 μL/well of diluted Matrigel® (100μg/mL). The plate was placed in a 37° C incubator, 5% CO2 to allow the biomatrix material to polymerize for 2 hours to study MRC-5 cell invasion. After 2 h the plate was removed from 37° C and Cell Seeding Stoppers were placed. One well without a Cell Seeding Stopper served as the positive control. MRC-5 cells (100 μL/well, 100,000–400,000 cells/mL stock) were seeded into each well of the plate. The seeded plate containing the Oris™ Cell Seeding Stoppers was incubated in a humidified chamber (37°C, 5% CO2) overnight to permit cell attachment. After the cells were attached to the matrices the spacers were removed. Media was removed and wells were washed with 100 μL of sterile PBS. After washing, 100 μL of culture media was added, and the cell plate was cooled to 4° C for 5 min. The top layer of the matrix was prepared on ice by diluting Matrigel®, 4-8 mg/mL. 50μL of the Matrigel® top layer was added. The addition of the two Matrigel™ layers ensured that the assay measures invasion, as opposed to 2D wound healing migration. The cell plate was then placed at 37° C for the biomatrix top layer to polymerize. After 30 min, 100 μL/well of additional media was added to each well and the plate was incubated in a humidified chamber (37°C, 5% CO2) to permit cell invasion. Cells were examined microscopically after 24, 48, and 72 h to monitor the progression of invasion. Three independent biological experiments were performed for statistical analysis using Student’s t-test. The images that were taken, were quantitated by WimScratch (Wimasis Image Analysis).”
Credit: N.F. Theodoroula et al. 2023
Their observations revealed that MRC-5 cells invaded the Matrigel in a time-dependent manner, further supporting the findings and highlighting the potential role of fibroblasts in angiogenesis.
In essence, this study pioneers the transformative idea that MRC-5 lung fibroblasts can be directly converted to ECs. This innovation was made possible by the critical role played by the Oris Universal Cell Migration Assembly kit, a tool which helped the researchers to examine and quantitatively analyze the cell invasion rate. It enabled the study of the behavior of these cells in a way that was not previously possible.
The Future of Vascular Tissue Engineering
In conclusion, the potential role of fibroblasts in angiogenesis and their direct conversion to ECs is a groundbreaking discovery. Thanks to the pivotal role of the Oris Universal Cell Migration Assembly kit in this research, scientists are one step closer to creating therapeutic modalities that can overcome the challenges of the past, exploring the potential of fibroblasts as a new cell source for the formation of vascular networks in engineered three-dimensional tissues in vitro.
This research reinforces the value of innovative tools like the Oris Universal Cell Migration Assembly kit in pushing the boundaries of what we know and what we can achieve in medical research. It’s an exciting time as scientists continue to uncover the hidden potentials of cells like fibroblasts in the quest for innovative, effective medical solutions.